×
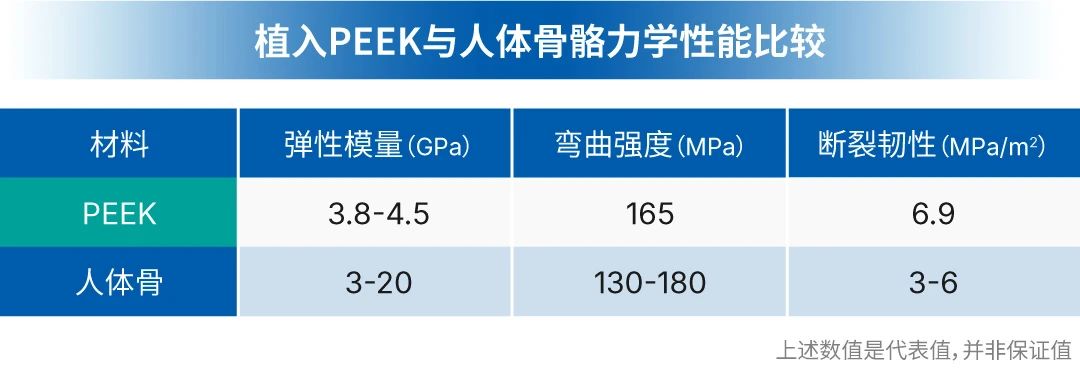
In the 1980s, a material called PEEK, due to its high similarity in elastic modulus to human cortical bone, quietly entered the vision of orthopedic doctors.
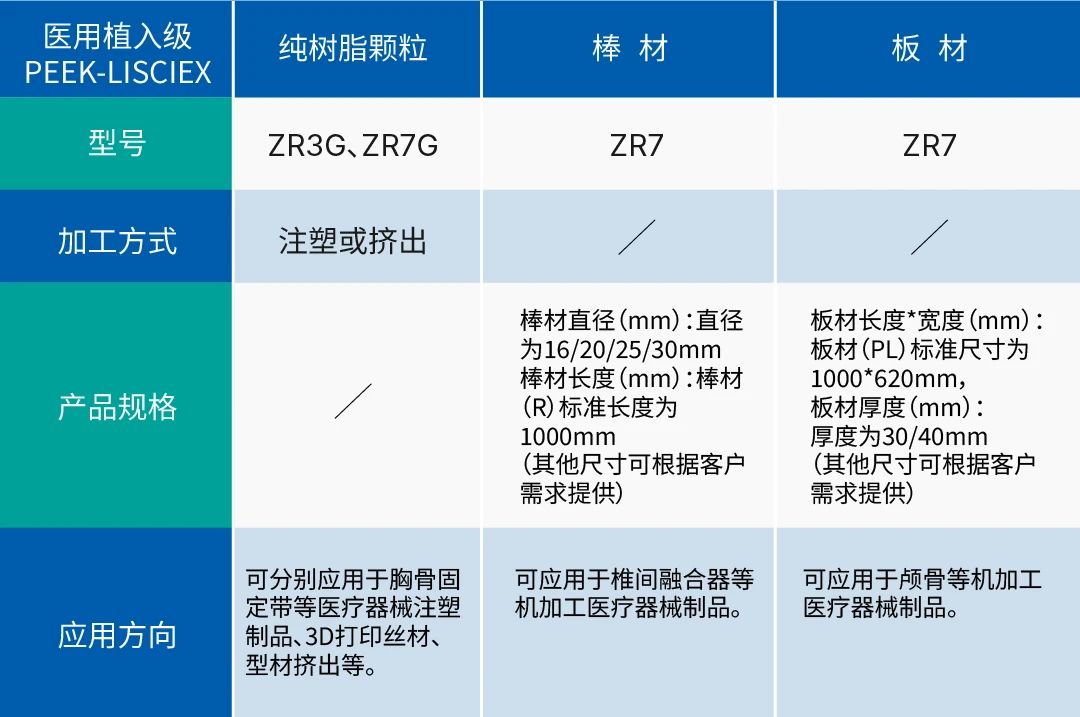
ZYPEEK has been deeply involved in the PEEK field for over ten years. In 2022, the medical implant grade PEEK-LISCIEX (Life Science X) series products were launched, successfully extending the application of PEEK from industrial applications to medical applications, achieving a domestic breakthrough in medical implant grade polyether ether ketone.

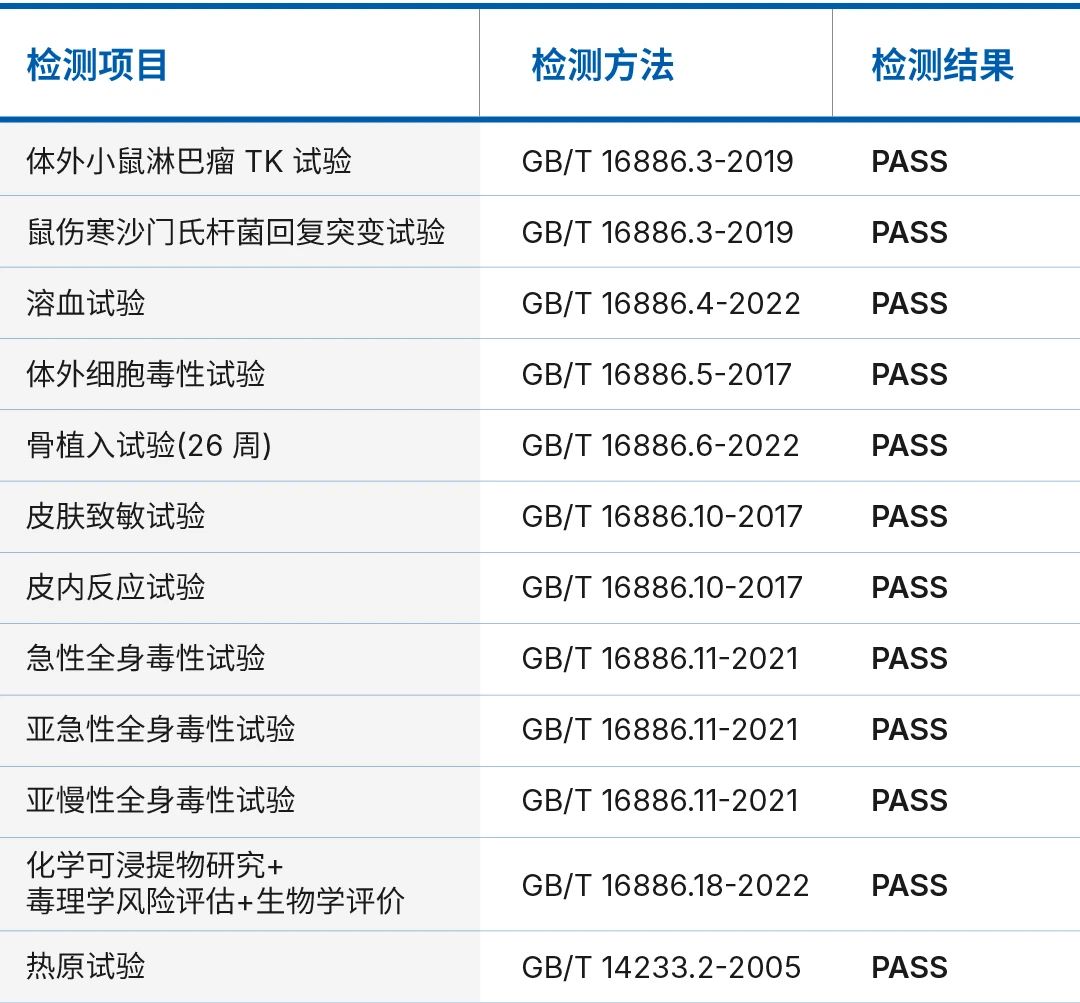
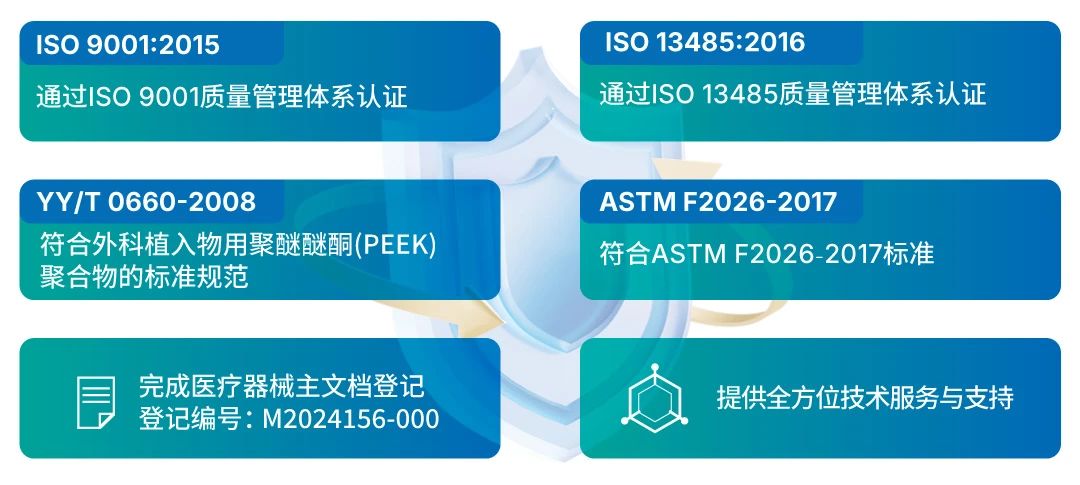
Biocompatibility is an important indicator for medical implant grade products. ZYPEEK has a strict quality system, comprehensive testing methods and processes, advanced testing equipment, high-standard production environment, and has been evaluated and certified by authoritative third-party institutions.

We deeply understand the importance of product quality. In every production process, we implement strict quality control, aiming to provide safe and reliable medical implant grade products to our partners through stringent testing standards.
Product Features and Advantages
Product Features

The product has the characteristics of "thermal stability, batch stability, color consistency, and high purity", which are the foundations for achieving batch stability and mass production of medical implant grade PEEK products. ZYPEEK strictly controls the quality of raw materials and quality control during the process to achieve the excellent quality of medical implant grade PEEK-LISCIEX products.

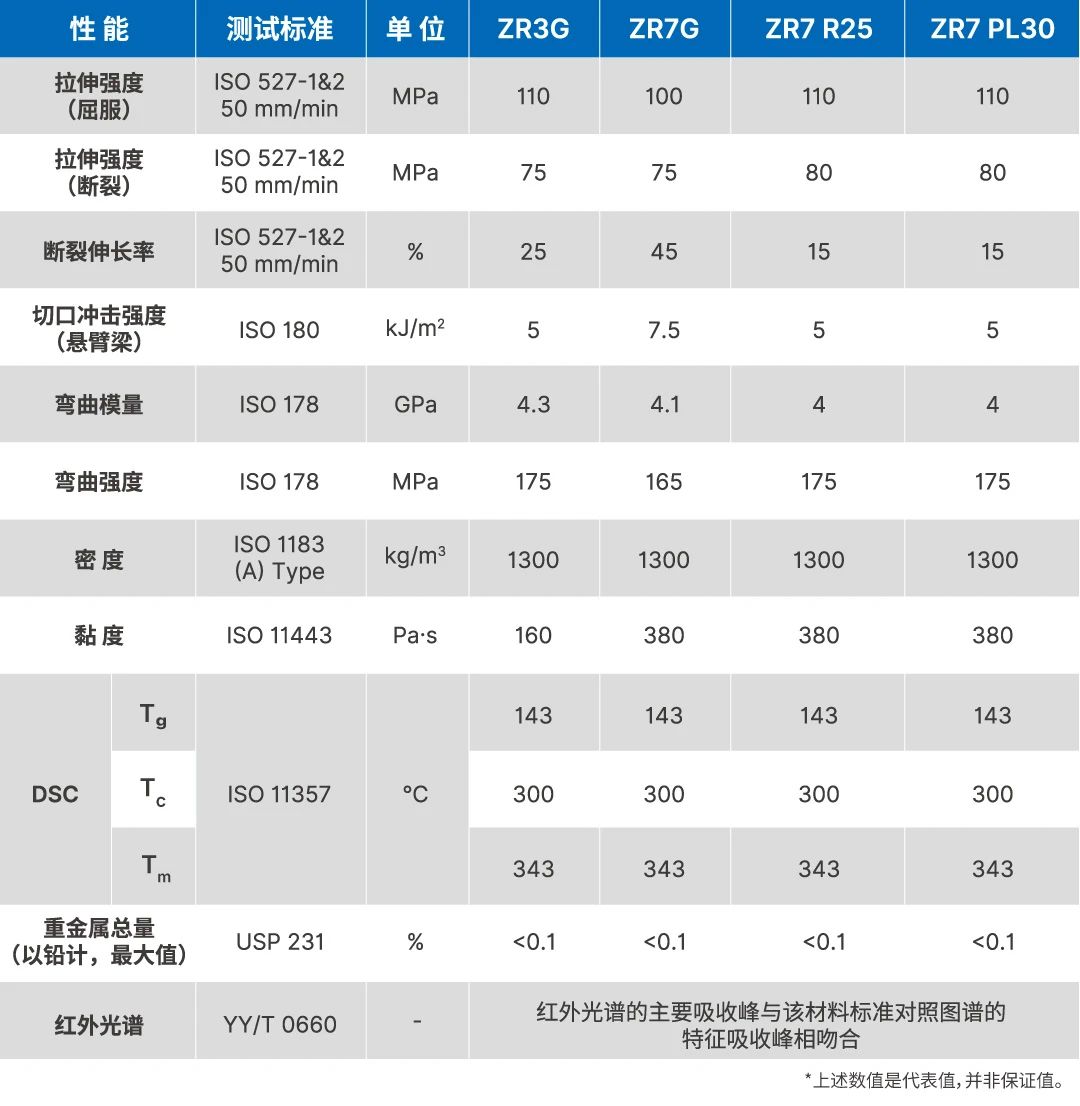
There are certain differences between industrial grade PEEK and medical grade PEEK in multiple dimensions.

According to Article 30 of the "Regulations on the Supervision and Administration of Medical Devices", engaging in medical device production activities should meet the following conditions:
Have production sites, environmental conditions, production equipment, and professional technical personnel suitable for producing medical devices;
Have institutions or dedicated inspection personnel and inspection equipment that can perform quality inspections on produced medical devices;
Have a quality management system that ensures the quality of medical devices;
Have after-sales service capabilities that are compatible with the production of medical devices;
Meet the requirements of product development and production process documents.

ZYPEEK also references the relevant requirements of medical devices in the production of PEEK materials:
Established a 10,000-grade purification production workshop, equipped with a professional technical and management team;
Referenced the inspection item requirements in YY/T 0660, established a professional quality inspection department, and equipped corresponding inspection equipment;
Formulated and implemented strict quality management systems in accordance with ISO 13485 requirements;
Leveraging Zhongyan's rich technical service experience in the PEEK processing field, provided comprehensive services to medical device production enterprises;
Completed the formulation of design development processes and production process standards, and standardized documentation according to ISO 13485 requirements.

Simultaneously, considering the project requirements of medical devices, ZYPEEK has completed evaluations of the impact of sterilization processes on the performance of PEEK; shelf life evaluations; toxicological evaluations; and batch stability verification, among others.
Differences between industrial grade PEEK and medical implant grade PEEK can be analyzed from dimensions such as production environment, management system, purification processes, purity control, product standards, and product packaging. The specific content is as shown in the figure:

On December 5, 2023, Xi'an Kangtuo Medical Technology Co., Ltd. obtained the Class III "Medical Device Registration Certificate". Its "Polyether Ether Ketone Sternal Fixation Band" was made using PEEK-LISCIEX products produced by ZYPEEK This collaboration not only marks a significant breakthrough for ZYPEEK in promoting domestic medical implant grade materials but also provides a solid material foundation for the research and development and manufacturing of domestic medical devices.

Implant grade PEEK can be used to prepare "implant devices" and is commonly used in orthopedic implant consumables (such as intervertebral fusion devices, ligament repair anchors, joint interface screws), neurosurgery repair patches (such as artificial skulls, maxillofacial bones), cardiovascular products (such as heart valves, pacemaker housings, etc.).
*The aforementioned applications of PEEK are its conventional application range and do not mean that all of Zhongyan PEEK has entered these application ranges.

ZYPEEK looks forward to working with partners to jointly promote the innovative application of medical implant grade PEEK materials in the field of life sciences; to share the development opportunities of the localization of medical implant grade PEEK materials, and to create limitless possibilities in the field of life sciences together!
If you need further information about ZYPEEK's products or wish to obtain samples, please feel free to contact 4001861177.
+86-(0431)-89625588